Defense against infection
o-4 hours – preformed mediators e.g. stomach acid, small molecules in secreted fluids
4-96 hours – recruitment of innate immune cells, primarily phagocytic = neutrophils & macrophages, results in pus
4-7 days – adaptive immune response from lymph nodes, results in swollen lymph nodes
The majority of infections get in via the mucosa
Bacteria – unicellular organisms, cell wall and plasma membrane but no intracellular membrane-bound organelles.
Viruses – essentially nucleic acids surrounded by protein coat
leukocytes = neutorphils, eosinophils, basophils (3 with lobed nuclei), lymphocytes, monocytes. Blood also contains platelets and erythrocytes.
Neutrophils – phagocytosis and releasing vasodilators, chemotaxins, etc involved in inflammation
Basophils – releasing histamine and other chemicals involved in inflammation
Eosinophils – destroy multicellular parasites and participate in immediate hypersensitvity reactions
Monocytes – phagocytose, secrete toxic chemicals, process and present antigens to CD4 T cells, secrete cytokines in acute phase response. Differentiate into macrophages in tissue.
Lymphocytes – recognition cells –
B Cells mature in bone marrow, activated in peripheral lymphoid organs, always display on their plasma membrane copies of the particular antibody its plasma cell progeny can produce. Huge variation in immunoglobulins because the DNA in each of the genes that code for immunoglobulin antigen binding sites is cut into small segments, randomly rearranged along the gene, and then rejoined to form new DNA molecules. Unstimulated B cell has huge nucleus and very little else, very small compared to erythrocyte, covered in antibody molecules. During activation, B cells are transformed into plasma cells which secrete antibodies (blood is full of plasma cells). Present antigen to CD4 T cells.
T cells mature in thymus, activated in peripheral lymphoid organs. T cell receptors for antigens are two chained proteins that, like immunoglobulins, have specific regions that differ from one T cell to another. However T cell receptors remain embedded in the cell membrane and are not secreted like immmunoglobulins. As in B cells, multiple DNA rearrangement results in millions of distinct clones. For T cells this rearrangement occurs in the Thymus. CD8 T cells bind to antigens on plasma membrane of target cells and destroy cells. Needs MHC II, found only on surface of macrophages, B cells and dendritic cells – APCs. CD4 T cells secrete cytokines that help to activate B, CD8 & NK cells and macrophages.
NK cells mature in bone marrow, bind directly and nonspecifically to virus-infected cells and cancer cells and kill them, and function as killer cells in antibody-dependent cellular cytotoxicity (ADCC).
Dendritic cells phagocytose pathogens, digest and combine proteins with MHC II to present to B and T cells (B & T cells exist in slightly different places in lymph nodes).
IgM – first antibodies to respond to infection, pentamer structure
IgG – numerous, main antibodies in blood
IgA – dimers, found in mucosa in GU, GI and respiratory tracts and in saliva
IgE involved in allergies (which affect ~25% of people) main function is thought to be defending against parasites
IgI sits on surface of B cells, never secreted, function unknown.
Antibodies can block binding of pathogens and toxins, act as opsonins for phagocytosis by neutrophis, and activate complement cascade which kills bacteria
Macrophages also present antigens to CD4 T cells, and secrete cytokines in acute phase response.
IL-1, TNF & IL-6 – stimulate IL-2 & IL-2 receptor expression, induce fever, stimulate systemic responses to inflammation, infection & injury
IL-2 stimulates proliferation of leukocytes & promotes conversion to plasma cells
Interferons stimulate cells to produce antiviral proteins
Chemokines are formed by damaged cells and facilitate the accumulation of leukocytes at sites of injury and inflammation
CSF stimulates proliferation of neutrophils and monocytes.
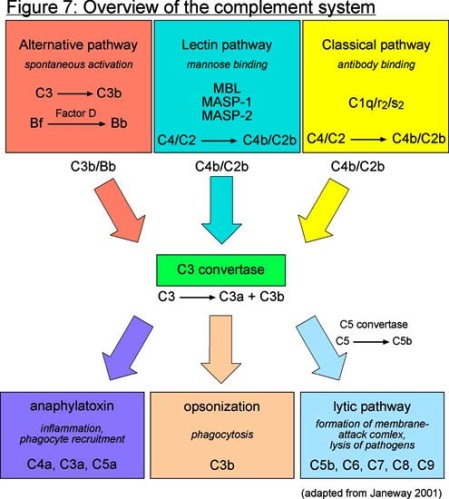
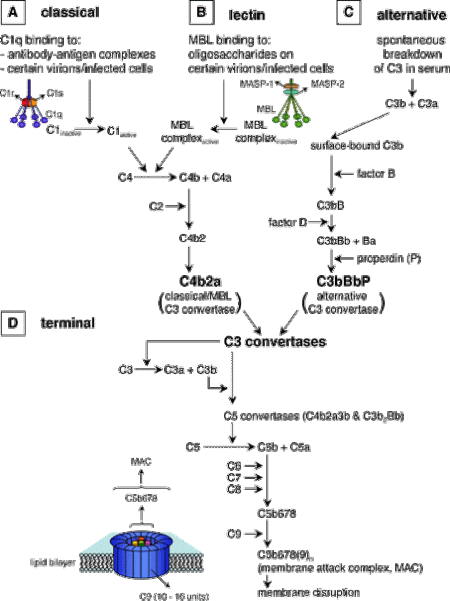
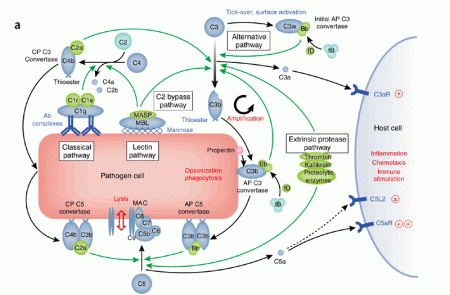
Inflammatory response – Entry of bacteria to tissue stimulates release of chemicals which cause vasodilation and increased permeability, movement of leukocytes from venules to interstitial fluid, known as chemotaxis (chemokines, a subset of cytokines, function as chemoattractants for distinct subsets of leukocytes). Bacteria killed by phagocytosis aided by opsonin, and by complement. Final stage of inflammatory response is tissue repair – fibroblasts divide rapidly, secrete collagen, angiogenesis occurs, then remodelling.
Systemic responses to infection – responses of tissues and organs distant from the site of infection or immune response – are collectively known as the acute phase response. All of these responses are elicited by one of more of the cytokines released from activated macrophages and other cells, in particular IL-1, TNF and IL-6, all of which serve local immune roles as well as acting hormonally to elicit distant responses.
Complement – Some of the activated complement molecules along the cascade cause, either directly or indirectly, vasodilation, increased microvessel permeability to protein, and chemotaxis. Also , one of the complement molecules, C3b, acts as an opsonin to attach the phagocyte to the microbe.
Nonspecific inflammation utilises the alternate complement pathway, which is not antibody-dependent and bypasses C1.
Positive selection in thymus selects for T-cells that are self-MHC restricted by only giving survival signals to B cells that bind to MHC on thymic cortical cells.
Negative selection in cortico-medullary junction of thymus, dendritic cells present self-antigen peptides and those T-cells which bind with enough affinity get apoptosis signals.
However some self-antigens are not present in thymus and can’t travel there in bloodstream, therefore need peripheral tolerance = clonal anergy – a T cell which receives signal from TcR binding antigen+MHC, but does not receive co-stimulation, is rendered anergic
Thymus is in upper part of chest, large at birth, continues to grow until puberty, then gradually atrophies and is replaced by fatty tissue. Consists mainly of mature lymphocytes
Lymph node is a honeycomb of lymph filled sinuses with large clusters of lymphocytes (the lymphatic nodules) between the sinuses. They also contain many macrophages and dendritic cells
Blood percolates through the vascular meshwork of the spleen where large collections of lymphocytes, macrophages and dendritic cells are found
Tonsils and adenoids are a group of small rounded lymphoid organs in the pharynx. They are filled with lymphocytes, macrophages and dendritic cells, and they have openings (crypts) to the surface of the pharynx.
MRSA – staph aureus – pyogenic Gram positive cocci that form clusters like bunches of grapes, cause skin lesions, abscesses, sepsis, ostomyelitis, pneumonia, endocarditis, food poisoning, TSS. Staph have protein A on surface, allowing them to escape antibody-mediated killing by binding Fc portion of immunoglobulins.
10^14 cells/person but only ~10% of these are ours. Rods = bacili, spheres = cocci. blue/purple stain = Gram positive – big peptoglycan cell wall soaks up and keeps lots of stain, survives in dry areas. red stain = Gram negative – thin cell wall covered by outer membrane, loses blue stain and takes up counterstain. survives in wet areas. Spores survive for ages in dry areas, e.g. C.difficile. Antibiotics, e.g. penicillins = beta lactan to get to penicillin binding protein to make cell wall leaky. Bacteria resist antibiotics by: stopping them getting in (especially gram- with outer membrane), eject ab back out; change its PBP so ab cant bind; beta-lactamases to break the ring of beta-lactam
pus = neutrophils, which have come to site of infection, phagocytosed bacteria, then died. Pus sometimes green because of myeloperoxidase, an intensely green antibacterial protein produced by some types of white blood cells. Blue-green pus found in certain infections of Pseudomonas aeruginosa as a result of the pyocyanin bacterial pigment it produces; amoebic abscesses of the liver produce brownish pus
HIV
http://www.unaids.org – 77000 people living with HIV in uk, of whom 22000 are women; figures for children not available. 0.2% prevalence among 15-49 year olds.
In UK population census 2001 white = 92.1%, black african = 1%, black carribean = 0.8%
In HPA 2007 figures for HIV+ patients accessing health care white = 54% (2/3 men), black african = 36% (2/3 women), black carribean = 9%
Under Disability Discrimination act 2005, it is unlawful to discriminate against people with HIV in the workplace, from the point of diagnosis. You don’t have to tell your employer unless you are a healthcare worker doing ‘invasive procedures’. Can ask for ‘reasonable adjustments’.
There is a near perfect correlation between HIV infection and disease, and drugs that lower the amount of virus in a patient (viral load) prevent opportunistic infections.
[Immunology – Kindt, Goldsby, Kuby & Osborne 5th Ed]
Each HIV virion expresses 72 glycoprotein projections composed of gp 120 (serves as a viral receptor for CD4 on host cells) & gp41 (transmembrane molecule). The viral envelope derives from the host cell and contains some of the host cell membrane proteins including class 1 and class II MHC molecules. The viral core has a layer of p17 and within that a layer of p24. The genome is 2 copies of single stranded RNA associated with 2 molecules of reverse transcriptase, and p32 & p10
HIV infects CD4+ T cells, some strains can also infect monocytes and other CD4+ cells. High affinity interaction between CD4 and coat protein of HIV-1, together with coreceptors – T-trophic strains of HIV preferentially infect T cells using CXCR4 coreceptor, while M-trophic strains preferentially infect macrophages using CCR5 coreceptor. Some chemokines can bind to CXCR4 & CCR5, blocking viral entry to cell. Some cytokines induce greater expression of chemokine receptors on cell surface, making more susceptible to HIV viral entry. Once virus enters cell, RNA reverse transcribed and cDNA copy (provirus). The gag proteins of the virus are cleaved by the viral protease into the forms that makeup the nuclear capsid in a mature infectious particle. Different stages in this viral replication process can be targetted by antiviral drugs.
HIV-1 infection of T cells with some HIV strains leads to formation of giant cells or synctia – fusion of a group of cells by interaction of viral gp120 on surface of infected cells with CD4 & coreceptors on other cells. Cell adhesion molecules weld cells together in a large multinuclear mass which eventually bursts. Formation of syncytia may be blocked by antibodies to some of the epitopes of the CD4 molecule, by genetically engineered soluble CD4, and by antibodies to cell adhesion molecules. Isolates of HIV from different sources were formerly classified as syncytia-inducing (SI) or non-syncytium-inducing (NSI). T-trophic strains were generally SI and M-trophic strains generally NSI. More recent classifications are based on which coreceptor the virus uses.
The most commonly used test for HIV detects the presence of HIV antibodies in the serum – they generally appear after ~3 months, after which time the individual is said to have seroconverted to to be seropositive for HIV-1. Diagnosis of AIDS includes the presence of HIV antibodies or virus; greatly diminished numbers of CD4+ T cells (<200cells/mm3, down from ~1000cells/mm3), impaired or absent delayed hypersensitivity reactions, and occurence of OIs. The primary infection most commonly goes unnoticed, but may show as a glandular-fever-like illness for a few weeks, or as a persistent generalised lymphadenopathy (PGL) lymph node enlargement for 3 months or more with no evidence of infection. There follows a long chronic phase ~8 years with no or few symptoms. The period from infection to death averages 9-12 years. The first overt indication of AIDS may be opportunistic infection with Candida Albicans (persistent or nonresponsive to treatment) or persistent hacking cough (P.corinii infection of the lungs) – indicating a rise in viral load and concomitant drop in CD4+ T cells.
Understanding how the immune system holds HIV-1 in check during the chronic phase – a dynamic interplay between the virus and the immune system – can lead to design of effective therapeutic and preventative strategies. Initial infection event causes dissemination of the virus to lymphoid organs and resultant strong immune response from antibodies and CD8 T cells. This keeps the viral load to a near steady state – viral replication continues and viral cells are destroyed by the immune system.
Prevention of maternal acquired infant HIV infection by antiretroviral treatment. US standard treatment Zidouvadine (AZT) for several months prior to delivery and treatment for infant for 6 weeks after birth,reduces infection rate by up to 67%, but its expensive. Trial in Uganda of antiretroviral Nevirapine (viramune) single dose at onset of labour, and singe dose to baby 24-30 hours after birth, results better than AZT, 200 times cheaper than AZT, cheaper than an HIV test, and conforms to the reality of maternal health care in Kampala.
Cell cycle G0 G1 –> S –> G2 –> M –> G0/G1
Controlled by cyclins
Most cancers are caused by sporadic or somatic mutations.
Tumour suppressor genes include p53, BRCA1, BRCA2, APC, and RB1
p53 is involved in recognising DNA damage, instigating DNA repair, halting cell cycle.
Most oncogenes are mutations of certain normal genes called proto-oncogenes. Proto-oncogenes are the “good” genes that normally control what kind of cell it is and how often it divides.
HER2 is a receptor found on the surface of certain cancer cells. It is made by a specific gene called the HER2/neu gene. HER2 is a receptor for a particular growth factor called human epidermal growth factor. HER2 promotes the growth of cancer cells. Tumours that are HER2-positive tend to grow more quickly. Trastuzumab (Herceptin®) is a drug that sticks to the HER2/neu protein so that the growth of the cancer cells is slowed down. Also lapitinib (tykerb).
Tamoxifen – partial estrogen receptor inhibitor, less toxic than chemo and good long term results, recommended at least 2yrs treatment as adjuvant post-op, and probably at least 5 yrs, SSRIs interfere with its action, cholesterol lowering and protects against post-menopausal loss in bone density
There are three types of hormone therapy: tamoxifen, aromatase inhibitors (eg Arimidex) and pituitary down regulators (eg Zoladex).
Another type of hormone treatment for premenopausal women with ER+ cancer is to stop the ovaries from working with particular drugs or to remove the ovaries, so that they do not produce oestrogen – ‘ovarian ablation‘.
Bones
Cholecalciferol (vitamin D3) to Calcidiol (25(OH)D3) in blood, to Calcitriol (1,25(OH)2D3) in kidneys and other tissues and is the most potent steroid hormone derived from cholecalciferol. Calcitriol has powerful anti-cancer properties, and stimulates absorption of Ca and P from gut. Decrease in serum Ca++ triggers increase in PTH which triggers increase in 1,25Vit D production in kidneys which increases Ca++ absorption in the gut. PTH also increases resorption of Ca and decreases resorption of P in kidney. Corticosteroids act on gut so Ca resorption doesn’t increase, leading to PTH & 1,25VitD acting on bone to release Ca++. Corticosteroids also decrease osteoblast activity
Bones largely made of organic matrix (osteoid, mainly type 1 collagen) and mineral calcium hydroxyapatite. Collagen deposited as woven bone (random, strenth eaqual in all directions) replaced by stronger lamellar bone (orderly, layered). epiphyseal ossification center – resting zone – proliferative zone – hypertrophic sone – ossifying cartilage – in epiphyseal plate
Local collections of osteocytes, osteoblasts, osteocytes form ‘basic molecular unit’remodelling 10% of skeletol/year. Quiesence – resorption – reversal – formation – mineralisation – quiesence
Most bones formed as cartilate ‘anlage’ then ~8 weeks enchondral ossification begins in primary (shaft) and secondary (epiphysis) centres of ossification. A plate of cartilage enlage becomes trapped between centres forming growth plate. Chrondocytes in this plate responsible for growth
Osteoprogenitor cells – pluripotent mesenchymal stem cells found in vicinity of all bone surfaces, when stimulated by growth factors they produce osteoblasts.
Osteoblasts synthesise, transport and arrange matrix proteins and initiate mineralisation. Have receptors for PTH, Vid D, leptin, estrogen, cytokines, growth factors and extracellular matrix proteins, and express factors that regulate differentiation and function of osteoclasts. Surrounded by matrix become ostocytes, or become flattened and quiescent lining cells.
Osteocytes communicate via cytoplasmic processes in tiny tunnels called canaliculi. Control Ca2+ and phosphate levels, and perform mechanotransduction.
Osteoclasts from same hematopoietic progeintor cells as monocytes and macrophagesregulated by macrophage colony stimulating factor (M-CSF), IL-1, TNF. Mature multinucleated osteoclasts from fusion of cells bind to bone surface via integrins, proton pump creates acidic environment to remove mineral, and proteases digest organic component of bone.
RANKL expressed on osteoblasts and marrow stromal cells simulates RANK activating stranscription factor, needed for generation and survival of osteoclasts. OPG can block RANKL. M-CSF on osteoblasts needed for generation of osteoclasts.
As a bone is broken down, substances are released into the microenvironment that initiate its renewal – growth factors, cytokines, enzymes such as collagenase
Peak bone mass largely genetically determined – OPG, RANK/RANKL, estrogen receptors, MHC. T-score – bone mass density (BMD) compared to young healthy control – norm for diagnosing osteoporosis. Z-score – BMD compared to age matched control
Osteomalacia – loss of bone material (equivalent to rickets in kids)
Osteoporosis – loss of entire bone substance
Osteomyolitis – bone infection, usually bacterial
Osteoarthritis – joint condition, usually mechanical origin
Osteoporosis – senile (low turnover), postmenopausal (high turnover), idiopathic. Women fracture prone ~10 years earlier than men. Primary prevention targets years of bone maturation – encouraging intake of 800-1000mg calcium/day, increase in weight bearing exercise, reduction in tobacco, alcohol & caffeine use. Secondary prevention targets menopause & postmenopause – assessment of risk, diet, exercise, estrogen HRT, bisphosphonates (bind to bone and inhibit osteoclasts), selective estrogen receptor modulators, PTH, calcium, vit D
Lordosis – inward, kyphosis – outward/forward, scoliosis – sideways
Fractures – traumatic, nontraumatic, closed, compound (open to skin), comminuted (splintered), displaced. Hematoma – clotted blood fills fractrure gap, seals space, provides fibrin mesh – soft tissue procallus. Degranulated platelets and migrating inflammatory cells release PDGF, TGF-beta, FGF & interleukins, which activate osteoprogenitor cells in periosteum and medullary cavity and surrounding soft tissue, which in turn stimulate osteoclastic & osteoblastic activity. They then form trabeculae (cancellous bone) in medullary cavity creating bony callus.
self efficacy has major effect on performance. Cognitive impairment in elderly people often due to nutritional deficits, dehydration, over medication, depression,
Paracetamol, aspirin – reducing protaglandins
Codeine – binding to opioid receptors mimicking endorphins
Bendroflumethazide – diuretic and widens blood vessels, to reduce tension
Benzodiazepines e.g. diazepam – releases GABA neurotransmitter
Corticosteroids e.g prednisolone – supress inflamation and immune response, parto f feedback mechanism in immmune sustem, lower white cell counts and antibody formation
Vancomycin and teicoplanin – glycopeptide antibiotics used to treat MRSA infections
Bacteriophages to kill specific bacteria
E4Med
Tests
- ELISA
- Direct & indirect immunofluoresence
- Real-time polymerase chain reaction, also called quantitative real time polymerase chain reaction, is a laboratory technique based on the PCR, which is used to amplify and simultaneously quantify a targeted DNA molecule, used for rapid detection and identification of MRSA strains.
- Sensitive PCR assays for viral RNA to test for level of HIV virus load
- Osteoporosis cannot be reliably detected in plain radiograms until 30-40% of bone mass lost. Measurement of blood levels of calcium, phosphorous and alkaline phosphatase not diagnostic. ‘DEXA scan’ to check bone density – dual energy x-ray absorptiometry – one stream absorbed by soft tissue, other by whole body. Also quantitative computed tomography, and biopsy
Stats
- Mean is affected by outliers, median isn’t, Mode is only ever used in A Level maths
- Standard deviation measures average distance from the mean
- Standard error ???
- P-value is the probability of obtaining these results if the null hypothesis was true, i.e. look for tiny p-value which means very little evidence that this could occur if null hypothesis were true
- Confidence interval (CI) – 95% CI is the range of values we can be 95%confident contains the true population – takes into account the population size, so gives more information than a p-value